Dr. John Daws, principal engineer at Daws Engineering LLC in Phoenix, Ariz., addressed a number of the issues that are key to nitrogen inflation at the Tire Society’s 25th Annual Meeting and Conference on Tire Science and Technology in Fairlawn, Ohio, last month.
In his paper, “Nitrogen Inflation for Passenger Car and Light Truck Tires,” he proposes the use of the total oxygen passing through the tire over its lifetime as a metric for evaluation of various inflation schemes. This metric is developed for several of the popular, available nitrogen inflation purities using both air and nitrogen as a top-off gas. Here is a brief excerpt. To read Daws’ paper in its entirety, click here.
Nitrogen inflation has not been adopted by any vehicle manufacturers, nor has it been promoted by tire manufacturers, but nitrogen inflation for passenger car and light truck tires is widely promoted in the tire service industry.
Many claims are made by supporters of nitrogen inflation: lower inflation pressure loss rates (IPLR), lower rolling resistance, higher fuel economy, lower running temperature, improved wear, and reduced age-related degradation, to name a few. The normal reasoning presented is that the tire materials are significantly more permeable to oxygen than to nitrogen (some promotional documents even suggest that nitrogen will not leak out of the tire at all), while omitting the information that the partial pressure of nitrogen inside the tire is several times higher than that of oxygen.
Clearly, claims of lower rolling resistance and lower running temperature and improved wear have no hope of being scientifically valid, since these tire characteristics are controlled by the mechanical deformation of the tire’s hysteretic materials.
There have been several studies aimed at validating one or more of these claims, but the tire types and conditions tested have varied widely and were observed over a short time. The issues are complicated by the fact that tire service providers offering nitrogen do not all offer the same level of nitrogen inflation gas purity, and the maintenance of the inflation pressure in the tire is the responsibility of the vehicle owner.
Oxygen in, oxygen out
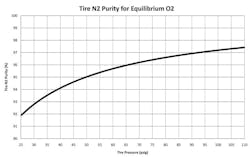
Since one of the objectives of nitrogen inflation is to minimize the permeation of oxygen through the tire, it follows that for every tire inflation pressure, there is a purity of nitrogen where the partial pressure of the oxygen inside the tire is equal to the partial pressure of oxygen in the atmosphere.
Figure 1 (see page 32) shows this relationship in graphical form. For a P-metric standard load tire with a maximum inflation pressure of 35 psi, the equilibrium nitrogen purity level would be about 93.5%. (For a load range E light truck tire with a maximum inflation pressure of 80 psi, that value increases to about 96.6%.)
Each type of tire has a certain partial pressure of oxygen at any nitrogen purity. It follows that if the nitrogen purity is such that the oxygen partial pressure inside the tire is less than that of the atmosphere, then oxygen will flow into the tire, and if the oxygen partial pressure inside the tire is greater than that of the atmosphere, oxygen will flow out of the tire.
Nitrogen purity over time
The initial nitrogen purity in the tire is obviously the primary concern of the tire service industry. However, from the standpoint of the consumer, the question becomes one of the stability of the benefits being purchased.
If the nitrogen purity established at the initial service degrades over time, then it follows that the benefit in inflation pressure loss rates would also degrade. Also, as NHTSA showed, if an air-inflated tire’s nitrogen purity increases with time, then why would the purchase of nitrogen inflation be of significant interest?
To explore these issues, one must not only look at the initial nitrogen purity in the tire, but also at the evolution in that purity over time. Obviously, the nitrogen purity of the top-off gas also becomes a variable in this analysis.
Some conclusions
The fact that a tire is about three times more permeable to oxygen than nitrogen is offset by the fact that the partial pressure of nitrogen is over three times higher than oxygen. This means that the initial permeation of gas out of an air-filled tire is almost half nitrogen and half oxygen. This also results in an evolution of the nitrogen purity in the tire over its service life.
The equilibrium partial pressure of oxygen is different for different maximum inflation pressures in different tire types. Higher purities of nitrogen may be obtained in fewer inflation steps with lower purity gas sources if the maximum inflation pressure for the tire is high.
Initial inflation of a tire with nitrogen at some purity level results in an initial reduction in inflation pressure loss rate. The IPLR approaches that of an air-inflated tire over time if the tire is topped off with air. The IPLR at any point in the tire’s life is linearly related to the level of nitrogen purity in the tire at that time. Since the nitrogen purity of a tire evolves over time, IPLR improvements are transient if the tire is topped off with air.
Initial inflation of a tire with nitrogen at some purity and topping it off with nitrogen results in another significant reduction in the amount of oxygen that flows across the tire during its lifetime.